Abstract

Introduction TP53m is observed in 5%-15% of newly diagnosed AML patients (pts) and represent a poor prognostic group that is highly refractory to available treatment, both intensive and non-intensive, including venetoclax-based therapies. Currently there are no established therapies specifically targeting TP53m in AML. Better understanding of the outcomes in TP53m AML pts with standard-of-care treatments can provide important insights for clinical management and drug development.
Methods Adult (≥18 years [yrs]) pts with new AML diagnosis from January 1, 2014 to February 28, 2021 were included from the nationwide Flatiron Health electronic health record-derived de-identified database. Prior to first line (1L) therapy start, all pts had TP53m determined from molecular tests or 17p del identified by cytogenetic/fluorescent in situ hybridization. Pts who received prior anti-leukemic therapies (except hydroxyurea, oral etoposide) or who had concurrent malignancies were excluded. Pts receiving 1L therapy were divided into 3 cohorts: hypomethylating agents (HMA; azacitidine/decitabine) and venetoclax (Cohort A), intensive chemotherapy (Cohort B), or HMA without venetoclax (Cohort C). Median overall survival (mOS) was estimated using Kaplan-Meier method. Baseline and clinical characteristics can impact treatment choice and survival in each cohort. Multivariate Cox proportional hazards models (including age, time to 1L therapy, comorbidities, TP53m status and cytogenic risk level, presence of 17p del, secondary and therapy-related AML, healthcare practice type, ECOG score, and post-1L allogeneic stem cell transplantation [allo-SCT]) were used to compare OS among treatment cohorts.
Results A total of 370 AML pts with either TP53m (n=124) or 17p del (n=166) or both (n=80) were included. Median age was 72 (range 24-84) yrs, and majority were male (59%) and white (69%). Most pts (85%) were from community oncology clinics. Overall median follow-up time for all pts was 6 (range 0-74) mos and was similar for the three cohorts (6, 7, and 5 mos for Cohort A, B, and C, respectively). Although cytogenetic risk category information was missing for more than one-third (34%) of pts, majority (65%) belonged to the ELN 2017 adverse cytogenetic risk category. Deletions in chromosomes 5, 7, or 17 were reported in 88% (n=324) of pts, with 68% having more than one deletion. Secondary AML was reported for 118 (32%) pts, of which 92 (78%) had prior myelodysplastic syndrome; 38 pts (10%) had therapy-related AML. Bone marrow (BM) blasts were ≤30%, 31-50%, and >50% in 41%, 24%, and 29% of pts, respectively.
Treatment Cohort A, B, and C included 94 (25%), 135 (37%), and 141 (38%) unique AML pts, respectively. Pts in Cohort A and C were older (median 75 and 77 yrs vs 62 yrs for Cohort B) and had more comorbidities (65% and 60% vs 40% in Cohort B with at least one comorbidity).
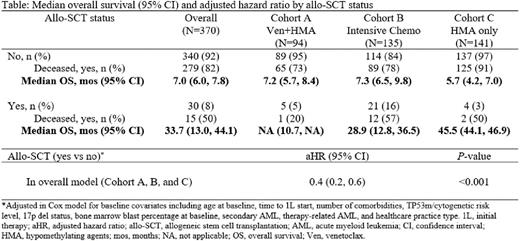
BM blast clearance post-1L was reported in 54% of pts (115/215) overall and 67% (38/57), 62% (68/110), and 19% (9/48), with median duration of blast clearance of 6.3, 6.9, and 5.4 mos for Cohort A, B, and C, respectively. Only 30 pts (8%) received allo-SCT (5%, 16%, and 3% in Cohort A, B, and C, respectively). Death during follow-up was reported in 80% (n=294) of pts with mOS of 7.3 mos (95% confidence interval [CI] 6.5-8.3). mOS estimate was 7.4 mos (95% CI 6.0-8.8) for Cohort A, 9.4 mos (95% CI 7.2-10.4) for Cohort B and 5.9 mos (95% CI 4.3-7.5) for Cohort C. Survival was much higher in pts receiving allo-SCT, which only few pts in each cohort received (Table). Addition of venetoclax to HMA (Cohort A) did not improve OS compared with HMA only (Cohort C) in an adjusted Cox model (adjusted hazard ratio [aHR]=0.9, 95% CI 0.7-1.3). In a similar analysis, no difference in OS was observed between Cohort A and B (aHR=0.9, 95% CI 0.6-1.4).
Conclusions In this large cohort of AML pts with TP53m/17p del, poor OS is observed with current standard-of-care treatment options. Pts treated with venetoclax and HMA combination did not have significantly different OS compared with pts treated with HMA only or pts receiving intensive chemotherapy. Younger age, fewer comorbidities, and higher number of pts receiving allo-SCT could have influenced apparent longer OS in the intensive chemotherapy cohort that was no longer significant in an adjusted model. Despite recent advances, TP53m AML pts continue to experience poor outcomes highlighting the significant unmet medical need and the need for novel therapies.
Disclosures
Daver:Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Servier: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Glycometrics: Research Funding; Arog: Consultancy; Celgene: Consultancy; Jazz: Consultancy; Novartis: Consultancy; Hanmi: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Trovagene: Research Funding; FATE Therapeutics: Research Funding; Amgen: Consultancy, Research Funding; Novimmune: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Syndax: Consultancy; Shattuck: Consultancy; Agios: Consultancy. Iqbal:Gilead Sciences, Inc.: Current Employment, Current holder of stock options in a privately-held company. Huang:Gilead Sciences, Inc.: Current Employment, Current holder of stock options in a privately-held company. Renard:Gilead Sciences, Inc.: Current Employment, Current holder of stock options in a privately-held company; Alphabet Inc.: Current holder of stock options in a privately-held company. Lin:Gilead Sciences, Inc.: Current Employment. Pan:Gilead Sciences, Inc.: Current Employment, Current holder of stock options in a privately-held company. Xing:Gilead Sciences, Inc.: Current Employment, Current holder of stock options in a privately-held company. Pan:Gilead Sciences, Inc.: Current Employment, Current holder of stock options in a privately-held company. Ramsingh:Gilead Sciences: Current Employment, Current holder of stock options in a privately-held company.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.